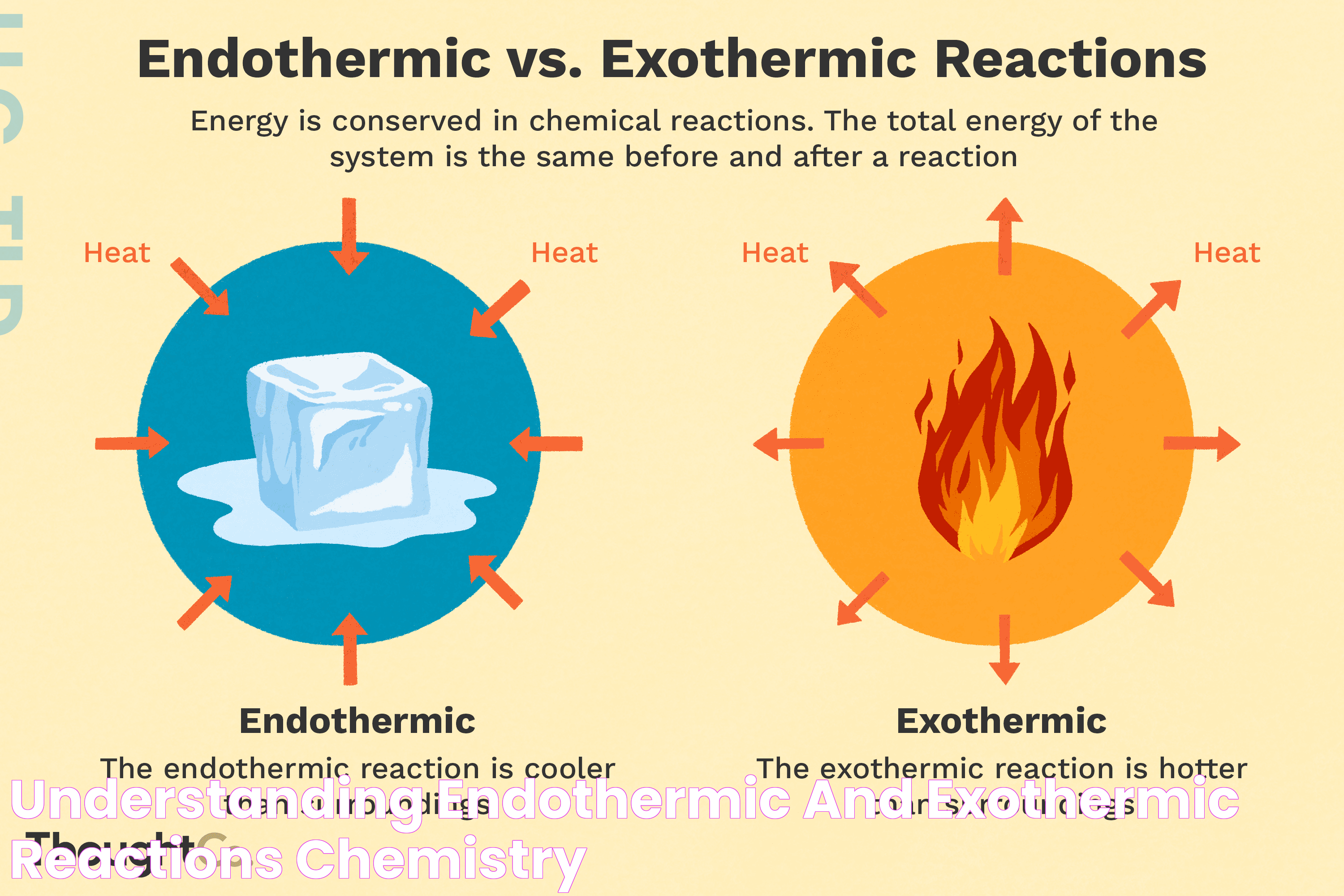
When delving into the fascinating world of chemistry, understanding the graphical representation of endothermic reactions is crucial. The question, "what does an endo thermic graph look like?" often arises among students and enthusiasts exploring thermodynamics. Endothermic reactions are those in which the system absorbs energy from its surroundings, typically in the form of heat. These reactions are characterized by a positive change in enthalpy, and their graphical representation provides a visual insight into the energy changes that occur during the reaction.
Endothermic graphs depict a unique pattern that distinguishes them from exothermic reactions. Typically, these graphs are plotted with the reaction progress on the x-axis and the energy level on the y-axis. The curve on the graph rises as the reaction progresses, illustrating the absorption of energy. This upward trend is indicative of the energy input required to drive the reaction forward. By scrutinizing an endothermic graph, one can gain a deeper understanding of the energy dynamics at play, which is essential for predicting reaction behavior and outcomes.
As we explore what an endo thermic graph looks like, it is essential to consider the broader implications of these reactions in various fields, including environmental science, engineering, and biology. Understanding these graphs not only aids in academic pursuits but also has practical applications in designing energy-efficient systems and processes. This article aims to provide a comprehensive guide to interpreting endothermic graphs, elucidating their characteristics, and highlighting their significance in real-world scenarios.
Read also:Tokyo Shampoo The Ultimate Hair Care Revolution
Table of Contents
- Introduction to Endothermic Reactions
- Understanding Endothermic Graphs
- How to Identify an Endothermic Graph?
- Key Characteristics of Endothermic Graphs
- What Does an Endo Thermic Graph Look Like?
- Endothermic Graph vs. Exothermic Graph
- Applications of Endothermic Reactions
- Real-World Examples of Endothermic Reactions
- How to Construct an Endothermic Graph?
- Common Misconceptions About Endothermic Graphs
- Analyzing Data from Endothermic Graphs
- The Role of Catalysts in Endothermic Reactions
- FAQs on Endothermic Graphs
- Conclusion: The Importance of Endothermic Graphs
Introduction to Endothermic Reactions
Endothermic reactions are a fundamental concept in chemistry that involve the absorption of energy. Unlike exothermic reactions, which release energy, endothermic reactions require an input of energy to proceed. This energy is typically absorbed in the form of heat, leading to a decrease in the temperature of the surroundings. The concept of endothermy is crucial for understanding various natural and industrial processes.
In endothermic reactions, the energy required to break the bonds of the reactants is greater than the energy released when new bonds are formed in the products. This results in a net absorption of energy. Common examples of endothermic reactions include photosynthesis, the melting of ice, and the evaporation of water. These reactions play a vital role in sustaining life and driving various biological and chemical processes.
The study of endothermic reactions is not only academically intriguing but also has practical implications. Understanding these reactions helps in designing energy-efficient systems, developing new materials, and improving industrial processes. By analyzing the energy changes involved in endothermic reactions, scientists and engineers can optimize processes and minimize energy consumption.
Understanding Endothermic Graphs
Endothermic graphs provide a visual representation of the energy changes that occur during an endothermic reaction. These graphs are essential tools for understanding the dynamics of energy absorption and the progression of the reaction. By examining an endothermic graph, one can gain insights into the energy requirements and the feasibility of the reaction.
The typical structure of an endothermic graph involves plotting the reaction progress on the x-axis and the energy level on the y-axis. As the reaction progresses, the graph shows an upward trend, indicating an increase in energy levels. This upward slope is characteristic of endothermic reactions and reflects the energy absorbed from the surroundings.
Endothermic graphs are useful for comparing different reactions and assessing their energy requirements. By analyzing these graphs, chemists can determine the most energy-efficient pathways and optimize reaction conditions. Additionally, endothermic graphs serve as valuable educational tools, helping students visualize and comprehend the concept of endothermy.
Read also:Affordable And Stylish Halloween Costume Ideas For All Ages
How to Identify an Endothermic Graph?
Identifying an endothermic graph involves recognizing specific features and patterns that distinguish it from other types of reaction graphs. The key characteristic of an endothermic graph is the upward slope, which indicates the absorption of energy. This upward trend is a visual representation of the energy input required for the reaction to proceed.
To identify an endothermic graph, look for the following features:
- An upward trend or slope as the reaction progresses from left to right.
- A positive change in enthalpy, signifying energy absorption.
- An initial dip or plateau, representing the activation energy barrier that must be overcome.
These features are indicative of an endothermic reaction and help distinguish it from exothermic reactions, which exhibit a downward slope due to energy release. By familiarizing yourself with these characteristics, you can easily identify endothermic graphs and interpret the energy dynamics involved.
Key Characteristics of Endothermic Graphs
Endothermic graphs possess unique characteristics that set them apart from other types of reaction graphs. Understanding these features is essential for accurately interpreting the energy changes and dynamics of endothermic reactions. The following are key characteristics of endothermic graphs:
Upward Slope
The most prominent feature of an endothermic graph is the upward slope. This upward trend indicates the absorption of energy from the surroundings as the reaction progresses. The slope represents the increase in energy levels, which is a defining trait of endothermic reactions.
Positive Enthalpy Change
Endothermic reactions are characterized by a positive change in enthalpy, which is reflected in the graph's upward slope. This positive change signifies that the energy absorbed is greater than the energy released, resulting in a net absorption of energy.
Activation Energy Barrier
Endothermic graphs often feature an initial dip or plateau, representing the activation energy barrier that must be overcome for the reaction to proceed. This barrier reflects the minimum energy required to initiate the reaction and is a crucial aspect of endothermic reactions.
By recognizing these key characteristics, one can effectively interpret endothermic graphs and gain insights into the energy dynamics involved in these reactions.
What Does an Endo Thermic Graph Look Like?
When visualizing an endothermic reaction, the graph typically exhibits a distinct pattern that is indicative of energy absorption. The question, "what does an endo thermic graph look like?" can be answered by examining the graph's key features and understanding their significance.
An endothermic graph is characterized by an upward slope, which represents the energy absorbed during the reaction. This upward trend is a visual depiction of the positive change in enthalpy, signifying that the energy input required to drive the reaction exceeds the energy released. The graph often starts with an initial dip or plateau, illustrating the activation energy barrier that must be overcome to initiate the reaction.
Overall, an endothermic graph provides a visual representation of the energy dynamics involved in the reaction. By understanding these features, one can gain insights into the energy requirements and feasibility of endothermic reactions.
Endothermic Graph vs. Exothermic Graph
Distinguishing between endothermic and exothermic graphs is crucial for understanding the energy changes involved in different types of reactions. While both graphs depict energy levels and reaction progress, they exhibit distinct patterns that reflect the energy dynamics of the respective reactions.
Endothermic graphs are characterized by an upward slope, indicating energy absorption. The positive change in enthalpy is reflected in the graph's upward trend, signifying that the energy input exceeds the energy released. In contrast, exothermic graphs exhibit a downward slope, representing energy release. The negative change in enthalpy is depicted by the graph's downward trend, indicating that the energy released is greater than the energy absorbed.
By comparing these graphs, one can gain insights into the energy dynamics and feasibility of different reactions. Understanding these differences is essential for predicting reaction behavior and optimizing reaction conditions.
Applications of Endothermic Reactions
Endothermic reactions have a wide range of applications in various fields, including environmental science, engineering, and biology. Understanding these reactions and their graphical representation is crucial for designing energy-efficient systems and processes.
In environmental science, endothermic reactions play a vital role in processes such as photosynthesis. During photosynthesis, plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This endothermic reaction is essential for sustaining life on Earth and maintaining the balance of the ecosystem.
In engineering, endothermic reactions are utilized in processes such as refrigeration and air conditioning. These reactions absorb heat from the surroundings, providing a cooling effect. By optimizing the energy efficiency of these processes, engineers can minimize energy consumption and reduce environmental impact.
In biology, endothermic reactions are involved in various metabolic processes, such as the synthesis of complex molecules. Understanding these reactions is crucial for developing new pharmaceuticals and improving healthcare outcomes.
Overall, endothermic reactions have significant implications in science and industry, driving innovation and sustainability.
Real-World Examples of Endothermic Reactions
Real-world examples of endothermic reactions provide practical insights into the energy dynamics involved in these processes. These examples highlight the significance of endothermic reactions in various fields and their applications in everyday life.
Photosynthesis
Photosynthesis is a classic example of an endothermic reaction. During this process, plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This reaction is essential for sustaining life on Earth and maintaining the balance of the ecosystem.
Melting of Ice
The melting of ice is an endothermic process that involves the absorption of heat from the surroundings. As ice absorbs energy, it undergoes a phase change from solid to liquid, resulting in the melting of ice. This process is crucial for understanding the behavior of water in different environmental conditions.
Evaporation of Water
The evaporation of water is another example of an endothermic reaction. During evaporation, water molecules absorb energy from the surroundings, allowing them to transition from a liquid to a gaseous state. This process plays a vital role in the water cycle and weather patterns.
These real-world examples demonstrate the significance of endothermic reactions in various fields and their practical applications in everyday life.
How to Construct an Endothermic Graph?
Constructing an endothermic graph involves understanding the energy dynamics of the reaction and accurately representing them on a graph. By following a systematic approach, one can create a visual depiction of the energy changes involved in an endothermic reaction.
To construct an endothermic graph, follow these steps:
- Identify the reaction progress and plot it on the x-axis.
- Determine the energy levels and plot them on the y-axis.
- Plot the initial energy level of the reactants.
- Include the activation energy barrier, represented by an initial dip or plateau.
- Depict the upward slope, indicating energy absorption and a positive change in enthalpy.
By following these steps, you can create an accurate and informative endothermic graph that visually represents the energy dynamics of the reaction.
Common Misconceptions About Endothermic Graphs
Endothermic graphs are often misunderstood due to common misconceptions about their characteristics and interpretation. By addressing these misconceptions, one can gain a clearer understanding of endothermic reactions and their graphical representation.
Misconception: Endothermic Graphs Show Energy Release
One common misconception is that endothermic graphs depict energy release. In reality, endothermic graphs represent energy absorption, as indicated by the upward slope and positive change in enthalpy. This misconception can be clarified by understanding the energy dynamics involved in endothermic reactions.
Misconception: Endothermic Graphs Are Always Linear
Another misconception is that endothermic graphs are always linear. While some endothermic graphs may appear linear, they often feature an initial dip or plateau, representing the activation energy barrier. Recognizing this feature is crucial for accurately interpreting endothermic graphs.
By addressing these misconceptions, one can gain a more accurate understanding of endothermic graphs and their significance in chemistry.
Analyzing Data from Endothermic Graphs
Analyzing data from endothermic graphs involves interpreting the energy dynamics and understanding the feasibility of the reaction. By examining the graph's features, one can gain insights into the energy requirements and optimize reaction conditions.
Assessing Energy Absorption
The upward slope of an endothermic graph reflects the energy absorption during the reaction. By analyzing this slope, one can determine the energy input required to drive the reaction. This information is crucial for optimizing reaction conditions and minimizing energy consumption.
Evaluating Activation Energy
The initial dip or plateau on an endothermic graph represents the activation energy barrier. By evaluating this feature, one can assess the minimum energy required to initiate the reaction. Understanding the activation energy is essential for predicting reaction behavior and optimizing reaction pathways.
By analyzing data from endothermic graphs, chemists can gain valuable insights into the energy dynamics of reactions and optimize processes for efficiency and sustainability.
The Role of Catalysts in Endothermic Reactions
Catalysts play a crucial role in endothermic reactions by lowering the activation energy barrier and facilitating the reaction process. Understanding the function of catalysts is essential for optimizing reaction conditions and improving reaction efficiency.
Catalysts work by providing an alternative reaction pathway with a lower activation energy. This allows the reaction to proceed more quickly and efficiently, reducing the energy input required. In endothermic reactions, catalysts can significantly enhance the reaction rate and minimize energy consumption.
By incorporating catalysts into endothermic reactions, chemists can optimize reaction conditions and develop energy-efficient processes. Understanding the role of catalysts is crucial for designing sustainable and environmentally friendly systems.
FAQs on Endothermic Graphs
Before concluding, let's address some frequently asked questions about endothermic graphs to enhance understanding and clarify common queries.
What is the main feature of an endothermic graph?
The main feature of an endothermic graph is the upward slope, which indicates energy absorption during the reaction. This upward trend reflects the positive change in enthalpy and distinguishes endothermic graphs from exothermic graphs.
How do endothermic graphs differ from exothermic graphs?
Endothermic graphs differ from exothermic graphs in their slope and energy dynamics. Endothermic graphs exhibit an upward slope, indicating energy absorption, while exothermic graphs have a downward slope, representing energy release.
Can catalysts affect endothermic graphs?
Yes, catalysts can affect endothermic graphs by lowering the activation energy barrier and facilitating the reaction process. This can result in a more efficient reaction with reduced energy input.
What are some common examples of endothermic reactions?
Common examples of endothermic reactions include photosynthesis, the melting of ice, and the evaporation of water. These reactions involve energy absorption and have significant implications in various fields.
Why is it important to study endothermic graphs?
Studying endothermic graphs is important for understanding the energy dynamics and feasibility of reactions. These graphs provide valuable insights into energy requirements and help optimize reaction conditions for efficiency and sustainability.
How can endothermic graphs be used in real-world applications?
Endothermic graphs can be used in real-world applications to design energy-efficient systems, optimize industrial processes, and develop sustainable technologies. Understanding these graphs is crucial for driving innovation and improving environmental outcomes.
Conclusion: The Importance of Endothermic Graphs
In conclusion, understanding what an endo thermic graph looks like is crucial for interpreting the energy dynamics of endothermic reactions. These graphs provide valuable insights into the energy absorption and feasibility of reactions, helping chemists and engineers optimize processes for efficiency and sustainability.
By recognizing the key characteristics of endothermic graphs, such as the upward slope and positive change in enthalpy, one can accurately interpret the energy changes involved in these reactions. This understanding is essential for designing energy-efficient systems, developing new materials, and improving industrial processes.
Overall, endothermic graphs serve as valuable tools for visualizing and understanding the complexities of endothermic reactions. By exploring these graphs and their implications, one can gain a deeper appreciation for the role of energy in driving chemical and biological processes.